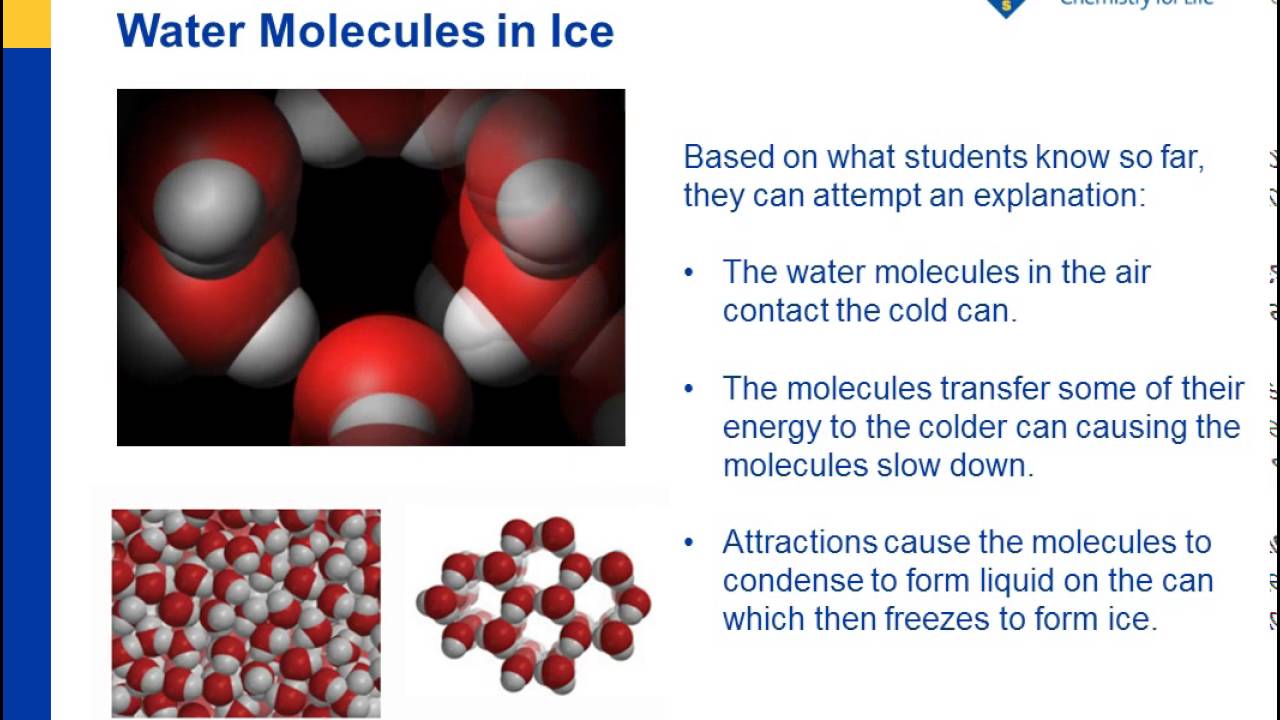
When Ice Melts What Happens To The Water Molecules . The molecules turn from a solid. How did the motion and arrangement of the water molecules change as the ice melted? The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. This is what happens when the ice cube (a solid) turns into water (a liquid). When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. As energy is transferred to the water molecules. As water cools, its molecular motion slows and the molecules move.
from ar.inspiredpencil.com
This is what happens when the ice cube (a solid) turns into water (a liquid). When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The molecules turn from a solid. As energy is transferred to the water molecules. As water cools, its molecular motion slows and the molecules move. How did the motion and arrangement of the water molecules change as the ice melted? Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure.
Freezing Water Molecules
When Ice Melts What Happens To The Water Molecules The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. How did the motion and arrangement of the water molecules change as the ice melted? The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. As energy is transferred to the water molecules. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. As water cools, its molecular motion slows and the molecules move. The molecules turn from a solid. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. This is what happens when the ice cube (a solid) turns into water (a liquid).
From www.naturphilosophie.co.uk
Changing States Fundamental Phases of Matter NaturPhilosophie When Ice Melts What Happens To The Water Molecules As water cools, its molecular motion slows and the molecules move. The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. The molecules turn from a solid.. When Ice Melts What Happens To The Water Molecules.
From sarahpetersonchemistry.blogspot.com
Summer Chemistry 2011 Activity 1 When Ice Melts What Happens To The Water Molecules When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure.. When Ice Melts What Happens To The Water Molecules.
From sciencing.com
What Happens to the Temperature of Ice As it Melts? Sciencing When Ice Melts What Happens To The Water Molecules As energy is transferred to the water molecules. As water cools, its molecular motion slows and the molecules move. The molecules turn from a solid. Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. This is what happens when the ice cube (a solid) turns into water (a liquid). When. When Ice Melts What Happens To The Water Molecules.
From lilahminschroeder.blogspot.com
What Happens When Ice Melts Into Water LilahminSchroeder When Ice Melts What Happens To The Water Molecules How did the motion and arrangement of the water molecules change as the ice melted? The molecules turn from a solid. As energy is transferred to the water molecules. Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. When ice melts, some of the hydrogen bonds are broken and the. When Ice Melts What Happens To The Water Molecules.
From studylib.net
What Happens to Molecules When a Substance Melts? Ice Liquid When Ice Melts What Happens To The Water Molecules The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules. When Ice Melts What Happens To The Water Molecules.
From teachsimple.com
Melting Ice Experiment Water Unit Journal by Teach Simple When Ice Melts What Happens To The Water Molecules As energy is transferred to the water molecules. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. This is what happens when the ice cube (a solid) turns into water (a liquid). The molecules turn from a solid. Ice melts when heat energy causes. When Ice Melts What Happens To The Water Molecules.
From www.youtube.com
What happens to level of water when Floating Ice containing metal piece When Ice Melts What Happens To The Water Molecules As water cools, its molecular motion slows and the molecules move. The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. As energy is transferred to the water molecules. The molecules turn from a solid. When an ice cube melts the water molecules obtain too much energy and break bonds that hold. When Ice Melts What Happens To The Water Molecules.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs When Ice Melts What Happens To The Water Molecules As energy is transferred to the water molecules. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. How did the motion and arrangement of the water molecules change as the ice melted? This is what happens when the ice cube (a solid) turns into. When Ice Melts What Happens To The Water Molecules.
From childhealthpolicy.vumc.org
💌 Which shape of ice cube melts the fastest. Which Ice Cube Shapes Melt When Ice Melts What Happens To The Water Molecules This is what happens when the ice cube (a solid) turns into water (a liquid). As energy is transferred to the water molecules. The molecules turn from a solid. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. When an ice cube melts the water molecules obtain too much energy and break. When Ice Melts What Happens To The Water Molecules.
From www.pinterest.com
The arrangement of water molecules in solid ice, liquid water and water When Ice Melts What Happens To The Water Molecules Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. As water cools, its molecular motion slows and the molecules move. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. When an ice cube melts the water molecules obtain too much energy and. When Ice Melts What Happens To The Water Molecules.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet When Ice Melts What Happens To The Water Molecules This is what happens when the ice cube (a solid) turns into water (a liquid). When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. The molecules turn from a solid. When ice melts, some of the hydrogen bonds are broken and the rigid crystal. When Ice Melts What Happens To The Water Molecules.
From www.sciencefacts.net
Water Expansion When Freezing When Ice Melts What Happens To The Water Molecules As water cools, its molecular motion slows and the molecules move. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. The hydrogen bonds in liquid water constantly. When Ice Melts What Happens To The Water Molecules.
From ar.inspiredpencil.com
Freezing Water Molecules When Ice Melts What Happens To The Water Molecules As water cools, its molecular motion slows and the molecules move. The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. As energy is transferred to the water molecules. The molecules turn from a solid. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat.. When Ice Melts What Happens To The Water Molecules.
From www.youtube.com
When Ice Melts, What Happens To The Water Level? 🤔 YouTube When Ice Melts What Happens To The Water Molecules When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. As water cools, its molecular motion slows and the molecules move. This is what happens when the ice cube (a solid) turns into water (a liquid). The molecules turn from a solid. Ice melts when heat energy causes the molecules to move faster,. When Ice Melts What Happens To The Water Molecules.
From www.snexplores.org
Explainer What are the different states of matter? When Ice Melts What Happens To The Water Molecules The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The molecules turn from a solid. As water cools, its molecular motion slows and the molecules move. How did the motion and arrangement of the. When Ice Melts What Happens To The Water Molecules.
From ar.inspiredpencil.com
Ice Cube Melting In Water When Ice Melts What Happens To The Water Molecules The molecules turn from a solid. As water cools, its molecular motion slows and the molecules move. Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like. When Ice Melts What Happens To The Water Molecules.
From www.britannica.com
Latent heat Definition, Examples, & Facts Britannica When Ice Melts What Happens To The Water Molecules Ice melts when heat energy causes the molecules to move faster, breaking the hydrogen bonds between molecules to form. How did the motion and arrangement of the water molecules change as the ice melted? The hydrogen bonds in liquid water constantly break and reform as the water molecules tumble past one another. This is what happens when the ice cube. When Ice Melts What Happens To The Water Molecules.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) When Ice Melts What Happens To The Water Molecules As energy is transferred to the water molecules. When an ice cube melts the water molecules obtain too much energy and break bonds that hold them together in a solid crystalline like structure. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. As water cools, its molecular motion slows and the molecules. When Ice Melts What Happens To The Water Molecules.